FizziCalc
Intermediate
Advanced
Cool Topics
Reference
Search
Games and Fun Stuff
Meeting Forum
Physics Links
Static Electricity |
|
Return to the Intermediate Level Page.
Static electricity studies the forces between stationary electric charges. Inside an atom, electrons carry a negative electric charge while protons carry a positive electric charge. Since protons are basically stuck inside the nucleus of an atom, the electrons that are in motion around the nucleus are usually the charge carriers. So, objects get charged mainly by gaining or losing electrons. Objects that have a positive charge have less electrons than protons. Objects that have a negative charge have more electrons than protons. If a object does not have an electrical charge, it is called electrically neutral.
Objects that have opposite charges are attracted to one another. Objects that have the same charges are repelled from one another. When two charged objects touch, the charge will be distributed evenly between them because electrons will flow from one object to another. Also, if two oppositely charged objects come in contact, some or all of their charges may cancel each other out.
An electrical ground is something capable of accepting or donating large number of electrons without significantly affecting its own electrical state. When an object is grounded, the object becomes neutral because it gives or receives electrons from the ground. The ground is so called because the earth can be an electrical ground. Since the earth is so big, it can give or accept electrons without affecting its own charge much.
Conservation of Charge
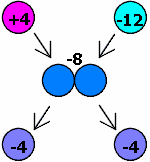 When two objects transfer charge from one another, the total charge of their system must be conserved.
In other words, the total charge of the two objects before must be the same as the total charge of the two objects after the transfer.
The illustration at right gives an example of this principle.
When two objects transfer charge from one another, the total charge of their system must be conserved.
In other words, the total charge of the two objects before must be the same as the total charge of the two objects after the transfer.
The illustration at right gives an example of this principle.We have one object with a charge of +4 and another object with a charge of -12. Their total charge is -8. When they touch, the charge is evenly distributed between each of them (electrons are transferred from the -12 to the +4). When they separate, their total charge still remains -8. Thus, the charges will each have a charge of -4.
Quantity of Charge and Coulomb's Law
The SI unit for electrical charge is the coulomb (C). All charges have discrete units. The smallest magnitude of electrical charge you can ever get normally is 1.6 x 10-19 C (this value is referred to as e). An object can have a charge of 2e or 3e, but it cannot have one of 2.5e. This is because the charge of an electron is -1.6 x 10-19 C. Similarly the charge on a proton is +1.6 x 10-19 C. You cannot have half an electron or half a proton.The coulomb was named for Charles Augustus Coulomb, who did experiments in 1785 on electrical charges. Charges can attract or repel, right? Well if they attract or repel, doesn't that mean that there is some kind of force between them? Coulomb devised a law to figure out the force between two charges:
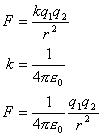
(Equation 2-1)
Look at the first line of the equation above. Doesn't it look a lot like Newton's law of gravitation? Just that the masses are replaced with charges (q1 and q2) and there is another constant (k) instead of G. The value of k is roughly 9.0 x 109 N m2/C2. Sometimes, instead of k, the equation is represented with another variable, e0, and the equation is written like it is on the third line. The second line shows the relationship between k and e0. e0 has a value of roughly 8.85 x 10-12 C2/N m2 and is refered to as the permittivity of free space. You don't have to worry about what e0 means in this section though. Using the equation, if you get a negative value for the force, the charges attract. If you get a positive value, the charges repel.
Electric Fields
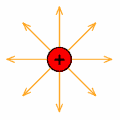
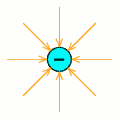 An electric field is a region where electrical force acts on a charge.
The direction of a field is always outward from a positive charge and inward towards a negative charge.
The illustration to the left shows the electric field of a positive charge.
The one to the right shows that of a negative charge.
Field lines show where a charge particle would move in an interaction with the field.
It is just another way of picturing the force between charges.
An electric field is a region where electrical force acts on a charge.
The direction of a field is always outward from a positive charge and inward towards a negative charge.
The illustration to the left shows the electric field of a positive charge.
The one to the right shows that of a negative charge.
Field lines show where a charge particle would move in an interaction with the field.
It is just another way of picturing the force between charges.The strength of the field is called the field intensity or field strength (E), which is the force per charge on a particular area:
(Equation 2-2)
For example, if an object with a charge of 3 x 10-6 C is acted upon by an electric force of 0.12 N, then the field strength at that point is 4 x 104 N/C.
So what happens to the field lines when two charges get near each other? Well, below are two examples.
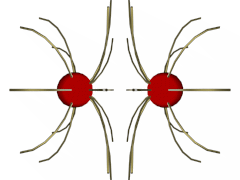
Same Charges (click on the illustration for a VRML model):
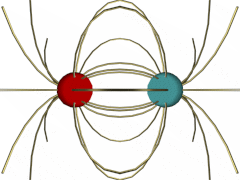
Opposite Charges (click on the illustration for a VRML model):
In the case of same charges, notice how the field lines are pushed away from each other. This is because field lines cannot cross. Thus, the two charges repel from one another.
In the case of opposite charges, you can see how the field lines originate from the positive charge and go into the negative charge. Field lines like to terminate at a negative charge. Thus, the two charges attract one another.
Electric Potential
If an electric field repels a charged particle from a certain direction, work must be done to move the particle in that direction. Electric potential is the work per unit charge that is needed to move that particle. For example, if work has to be done to move a positively charged particle from point A to point B in an electric field, point B has a higher potential than point A. If the particle "falls" from point B back to point A, it will be able to perform the same amount of work done to it.Potential difference or potential drop is the change in electric potential between two points. Potential drop (V) is given by:
(Equation 2-3)
The units for potential drop is the volt (V). This is why potential drop is sometimes called voltage.
The work done to move one elementary charge (e) across the potential difference of one volt is:
Now, 1.6 x 10-19 J is a pretty small number to be working with sometimes. So, physicists decided to call this value the electronvolt (eV):
Physicists use the electronvolt because its size is convienent for expressing the energies involved in some chemical reactions between atoms.
If you have a uniform electric field, you can also express the electric field intensity using potential drop:
(Equation 2-4)
d is the change in position in meters. This is why electric field strength is sometimes expressed in volts per meter instead of newtons per coulomb, though they are the same exact thing.